| Chemical Agent |
Action |
| Protein Denaturants |
|
| Heavy metals: |
|
| Silver: AgNO3 |
Protein precipitant activity neutralized by organic matter used in
eyes of newborns to prevent gonorrhea |
| Copper:
CuSO4 |
Kills algae in swimming pools and water supplies. Fungicide for plant
diseases, Bordeaux mixture |
| Mercury:
HgCI2 |
Combines with -SH groups in proteins Very toxic to humans Used to treat
syphilis with serious side effects. Organic mercury compounds still used.
e.g.. Mercurochrome |
| Halogens: |
|
Iodine
Tincture of iodine (0.2% I2 in 70% ethanol),
Iodine in solution of KI (KI3)
Iodophors
(I2 complexed with detergents)
|
Iodinates proteins containing tyrosine
residues
antiseptic on skin disinfectant of medical
instruments small scale water purification; relatively nontoxic on skin
but toxic internally
Betadene is an iodophore (Ps. aeruginosa
can live in/on iodophores)
|
| Alcohols: |
|
|
Ethanol and Isopropyl alcohol:
most
active with some water present (50-70% alcohol in water)
|
Lipid solvents and protein denaturants. Antiseptic on skin. Disinfectant
for hospital items |
| Aldehydes: |
|
|
Formaldehyde,
HCHO available as 37% solution in water
(formalin) used as 2% aqueous solution or as gas for fogging
|
Alkylating agent combines with -NH2, -COOH, and -SH groups in nucleic
acids and proteins. Neutralized by organic matter. Used for embalming of
corpses and the small amounts in wood smoke aid smoke to preserve meat |
|
Glutaraldehyde,
COHCH2CH2CH2CHO:
used as 2% aqueous
solution
|
Less toxic than formaldehyde. Cold sterilization of hospital goods
neutralized by organic matter |
|
Ethylene
oxide:
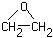
Used as a gas |
Alkylating
agent very toxic, used in special gas
sterilization
units for heat sensitive goods e.g. plastic disposable equipment
|
| Phenols: |
|
| Phenol,
carbolic
acid, C6H12OH used as 5% aqueous solution. Cresol & xylenols also used
|
Protein denaturant. Disrupts cell membrane at low concentrations. Very
toxic, activity increased by soaps, not affected by organic matter.
Disinfectant for large, dirty surfaces (Lysol)
Natural polyphenols (tannins) used to preserve hides (tanning) |
|
Hexachlorophene
a
chlorinated biphenol
|
Once widely used because it persists on skin. Restricted now due to
worry about brain damage. Excellent for control of Staphylococcus. |
| Detergents: |
|
Soaps
anionic detergents (-ve) |
Disrupt microbial membranes ( best for Gram -ve Anionic have only mild
action due to repulsion of -ve charged on bacteria and can neutralise many
antiseptics |
Quaternary Ammonium Compounds
cationic detergents (+ve) |
For effective but not against TB or spores. Used as sanitisers. |
| Oxidising Agents: |
|
| Hydrogen Peroxide (H2O2) |
Relies on 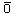 production
but not reliable production
but not reliable |
|
Chlorine:
In
Cl 2 gas. Ca(OCI)2 and NaOCl. the active ingredient is
HOCl, and 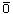 formed at neutral
or acidic conditions |
Activity is neutralized by organic mater, used in water purification
and general disinfectant in food and dairy industries |
Potassium permanganate
(KMnO4) Condies Crystals |
Strong oxidising agent. May irritate skin |
| Dyes & Others |
|
| Gention Violet
1% |
Inhibit G+ve bacteria and fungi not G-ve's |
| Acriflavin |
Inhibit G-ve bacteria |
| Brilliant Green and
Malachite Green |
Once used for gastro. Rarely used now. |
| Lime Ca(OH)2 |
Powerful disinfectant. Deactivated by CO2
Dilute solution is whitewash |
| Chlorinated lime (bleaching powder) |
Chlorine liberating disinfectant |
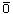 formed at neutral
or acidic conditions
formed at neutral
or acidic conditions